

DESIGN FMEA – MINIMISING RISK
We ensure risk is minimised through the design process by undertaking Design Failure Mode Effects Analysis, as part of the development process.

MEDICAL DEVICE STANDARDS
We ensure your products meet the required standards. We have associate regulatory specialists to undertake this for any medical device development.
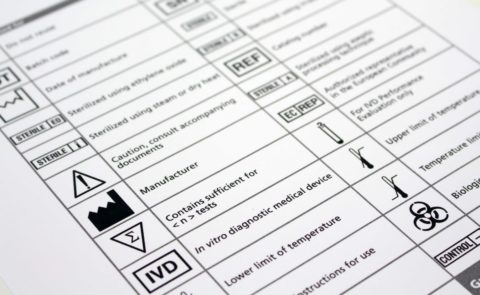
MEDICAL DEVICE SYMBOLS
We ensure the relevant symbols are displayed on your medical device. These will either be engraved on the plastic moulding or on a printed label.

REGULATORY PACKAGING LABEL
We can design your product label, ensuring the required information, such as medical device symbols and expiry dates are clearly shown